Wearable devices are revolutionizing the way we interact with our bodies. But are we fully exploring their potential? With precision medicine on the rise, it’s crucial to find more cost-effective and efficient ways to monitor key biomarkers (such as blood sugar for diabetes). And that’s where the real magic happens: there are already minimally invasive devices capable of monitoring glucose, blood pressure, heart rate, and even the number of steps we take. It’s time to ask ourselves: how can we use this information to further improve our health and quality of life?
Imagine being able to identify patterns in your biomarkers and receive real-time alerts when something is off. This could help prevent health issues before they become chronic and harder to treat. Nowadays, research in this field is pointing towards new markers, such as acute kidney injury markers, which still claim the lives of approximately 2 million people worldwide and affect 1 in 5 hospitalized patients. Among all the diagnostic targets and methods, there is one highly interesting marker: urea, a molecule associated with both renal failure and acutely decompensated congestive heart failure.
Current tests, while demonstrating accuracy, do not allow for real-time monitoring and are invasive due to the constant need for blood sample collection. This led researchers from the Laboratory of Genetics and Molecular Cardiology to seek an alternative, and the answer was in our sweat.
Urea and Sweat: What We Know So Far.
Urea is a substance produced by the liver as a result of protein metabolism. Under normal conditions, it is excreted in urine by the kidneys, but minimal amounts can also be eliminated through sweating. It is important to note that the amount of urea present in the blood is naturally higher than in sweat, however, in some pathological conditions such as Congestive Heart Failure (CHF), blood urea levels can increase, leading to cardiovascular complications and hospitalizations.
A study conducted by the Tokyo Women’s Medical University in 2017 found that patients with high levels of blood urea nitrogen had a greater risk of death from cardiovascular disease and hospital readmissions. For this reason, there is a growing interest in understanding the relationship between urea concentrations in blood and sweat.
In 2012, researchers from the Renal Research Laboratory at Manchester Royal Infirmary successfully tested the technique of transdermal reverse iontophoresis of urea to monitor urea levels. This technique proved to be safe and effective in discriminating between Chronic Kidney Disease patients and healthy individuals, as well as allowing for monitoring the decrease of blood concentrations in patients undergoing hemodialysis, all through sweat.
The race for biosensors
To develop a wearable device, at a minimum, the following are required:
Analytes: When there is an injury to an organ or abnormal functioning, certain molecules are sought in our laboratory tests. As mentioned in the previous study, Blood Urea Nitrogen (BUN) is our analyte, which can be altered in conditions that compromise its elimination due to other non-renal diseases. Other examples of analytes (biomarkers) include creatinine, glucose, cholesterol, pH, and hemoglobin, to name a few.
Natural or biomimetic elements: Often, analytes are not easily detected and biological or synthetic structures (biomimetic elements) are necessary. These types of elements facilitate recognition, amplifying the identification of analytes. Strategies vary and include nanoparticles, antibodies, and even new strategies such as molecularly imprinted polymers, which are artificially produced and replicate the topography of target molecules.
Signal detection and transduction mechanisms: At first glance, this may seem like a complicated term, but in summary, these mechanisms interpret signals from nature and convert them into values, usually numerical, to understand what is happening in our surroundings or in our body. In the context of renal injury, for example, there are four important biosensor examples: electrochemical, plasmonic, nanoparticle, and molecular probes. However, the world of biosensors goes far beyond this, with an extensive range of applications in various fields and a numerous variety.
The search for biosensors dates back to 1956, when Leland C. Clark, Jr. developed the “Father of Biosensors,” also known as the Clark electrode, to detect oxygen. Six years later, he created an amperometric enzyme electrode for glucose detection, which made an important contribution to the field. In 1969, Guilbault and Montalvo, Jr. discovered the first potentiometric biosensor for urea detection, and five years later, Yellow Spring Instruments (YSI) released the first commercial biosensor for blood glucose measurement. Since then, these devices have become increasingly compact and potentially wearable.
The biosensor developed at the University of são Paulo (USP)
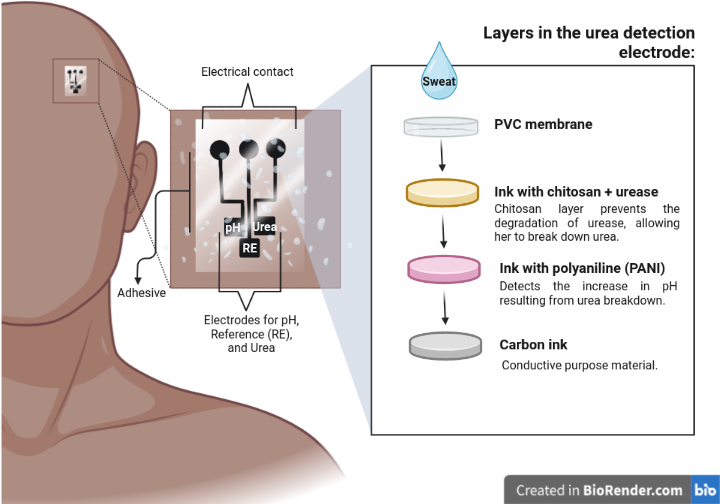
In 2022, two research groups joined the race to develop a wearable potentiometric device capable of measuring urea in sweat. One of the groups was led by the São Carlos Institute of Chemistry and the other by the Molecular Genetics and Cardiology Laboratory at INCOR. Both groups used the Screen-Printed Electrode Array technology to build the device, which was modified with Polyaniline (PANI) nanoparticles and Biotinta with urease, an enzyme that converts urea into Carbon Dioxide and Ammonia. During the tests, researchers were able to observe differences between those sensors modified with the enzyme (urease) and those without it. In sensors modified with urease, the potentiometric sensor was able to translate differences in urea concentration, which did not happen in other cases.
One of the challenges faced by researchers was to ensure the stability of urease. This enzyme is naturally unstable under certain conditions, such as high temperatures, pH variations, and concentrations of metallic ions. In addition, in human sweat, pH can vary due to various factors, including physical activity, diet, and overall health status. This required the device to be effective in detecting urea in sweat under different situations. It was necessary to ensure a stable and reliable response, regardless of the pH of the sweat, and the solution was to incorporate chitosan into one of the layers, thus protecting urease from degradation and improving the efficiency of the reaction with urea.
Thus, the researchers were able to measure urea levels in real sweat samples using a biosensor that was calibrated with artificial sweat solutions with a similar pH to the sample. The results showed an average urea concentration of 10.9 mM, which is consistent with previous reports for urea using wearable devices and is within expected levels for healthy individuals (below 60 mM).
From now on, the possibilities are endless. We can test this technology on a large scale and integrate it with other tools, such as cloud storage and the Internet of Things (IoT), and even consider the growing use of artificial intelligence (AI). In fact, today we can consider what was once unimaginable.
Other sources:
Bandodkar, Amay J., Wenzhao Jia, Ceren Yardımcı, Xuan Wang, Julian Ramirez, and Joseph Wang. 2015. “Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study.” Analytical Chemistry 87 (1): 394–98. https://doi.org/10.1021/ac504300n.
Derin, Esma, Fatih Inci, I. Palchetti, and M. Mascini. 2010. “Biosensor Technology: A Brief History.” Edited by Piero Malcovati, Andrea Baschirotto, Arnaldo d’Amico, and Corrado Natale. Sensors and Microsystems 7 (2): 15–23. https://doi.org/10.1021/acssensors.1c01781.
Ibáñez-Redín, Gisela, Giovana Rosso Cagnani, Nathalia O. Gomes, Paulo A. Raymundo-Pereira, Sergio A. S. Machado, Marco Antonio Gutierrez, Jose Eduardo Krieger, and Osvaldo N. Oliveira. 2023. “Wearable Potentiometric Biosensor for Analysis of Urea in Sweat.” Biosensors and Bioelectronics 223: 114994. https://doi.org/https://doi.org/10.1016/j.bios.2022.114994.
Jujo, Kentaro, Yuichiro Minami, Shintaro Haruki, Yuya Matsue, Kensuke Shimazaki, Hiromu Kadowaki, Issei Ishida, et al. 2017. “Persistent High Blood Urea Nitrogen Level Is Associated with Increased Risk of Cardiovascular Events in Patients with Acute Heart Failure.” ESC Heart Failure 4 (4): 545–53. https://doi.org/https://doi.org/10.1002/ehf2.12188.
Palchetti, I., and M. Mascini. 2010. “Biosensor Technology: A Brief History.” Edited by Piero Malcovati, Andrea Baschirotto, Arnaldo d’Amico, and Corrado Natale, 15–23.