With each new publication, our understanding of the genetic predisposition to diseases is becoming more sophisticated. At the start of the 20th century, the most advanced scientific evidence suggested that consuming large amounts of meat, eggs, and milk caused atherosclerosis in experimental animals, which is characterized by the accumulation of fats and other substances in the blood, and was soon associated with high cholesterol levels. Since then, science has made significant strides, leading to the development of numerous medical action guides, new ways of detecting hypercholesterolemia, and new treatments. In Brazil, the Longitudinal Study of Adult Health (ELSA), a long-term research project that began in 2008, is contributing to this new paradigm, particularly with regards to genetic predisposition to some diseases. The project is a collaborative effort among six Brazilian universities, including the University of São Paulo, Federal University of Rio Grande do Sul, Oswaldo Cruz Foundation (Fiocruz) in Rio de Janeiro, Federal University of Espírito Santo, Federal University of Minas Gerais, and Federal University of Bahia. As part of this partnership, the Laboratory of Genetics and Molecular Cardiology recognized the need to better understand how genetic predisposition is linked to the risk of developing certain cardiovascular diseases, with a focus on the PCSK9 enzyme. This research was conducted in 2021.
PCSK9, what is it?
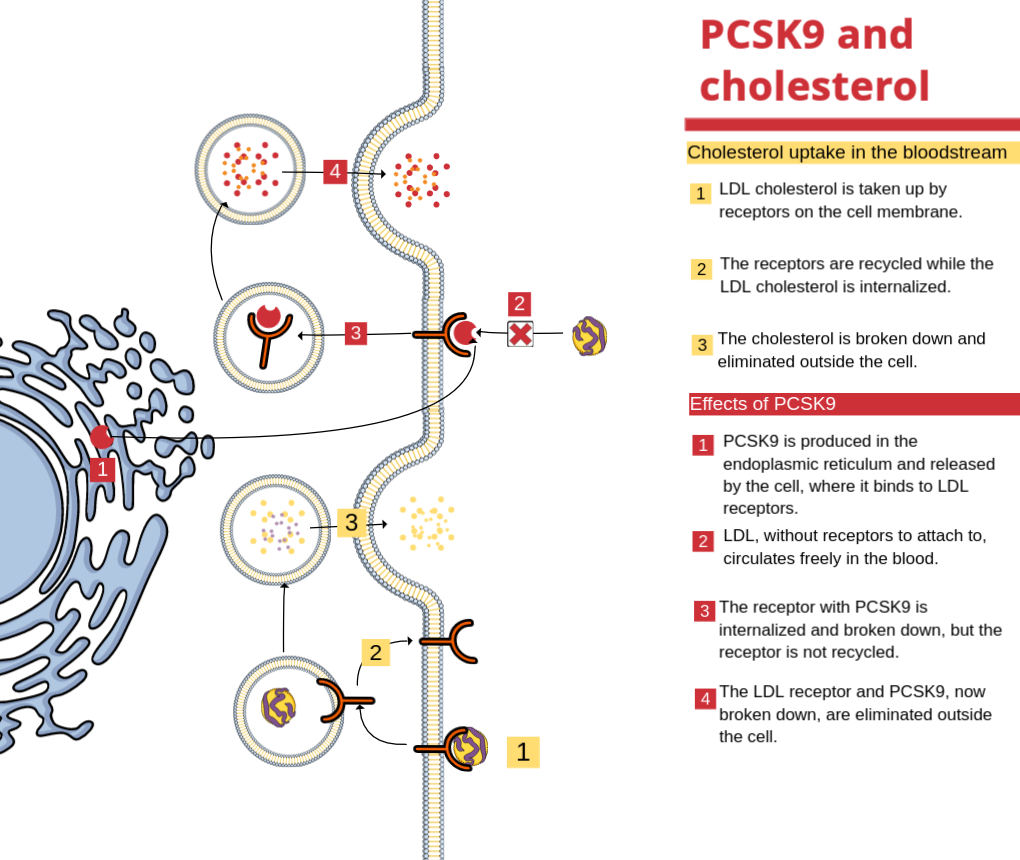
PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) is a protein that plays an important role in cholesterol metabolism. It is primarily produced in the liver and acts to regulate the degradation of LDL (Low-Density Lipoprotein) receptors, also known as “bad” cholesterol. These receptors are responsible for capturing circulating LDL cholesterol in the blood, reducing its quantity and thus preventing the accumulation of fat in the arteries. Under normal conditions, PCSK9 facilitates the internalization of LDL receptors into cells, where cholesterol is destroyed. However, when there is an excessive production of PCSK9, normal recycling of receptors is impeded, and more receptors are destroyed, thus increasing the amount of LDL cholesterol in the blood. The control of PCSK9 has been the subject of several studies and research aiming to develop new therapeutic strategies for the treatment of hypercholesterolemia, which is a condition characterized by high levels of cholesterol in the blood and one of the main risk factors for cardiovascular diseases. Blocking PCSK9 through monoclonal antibodies (proteins created in a laboratory) has shown to be a promising option, as it reduces the amount of PCSK9 in the blood and increases the recycling of LDL receptors, thus decreasing the amount of circulating cholesterol and preventing the accumulation of fat in the arteries. Regarding the mutation of the gene that contains the production instructions for this protein, it was first identified in a French family with familial hypercholesterolemia, according to a 2003 publication in the journal Nature Genetics. Since then, the scientific community has been interested in the subject, mainly because findings related to PCSK9 are generally quickly applicable in practice.Cardiovascular risk factors and PCSK9
As we have seen so far, PCSK9 has a close relationship with cholesterol, which we are fully familiar with as it relates to our lifestyle. However, even when we follow a healthy lifestyle and adopt measures to maintain adequate cholesterol levels, we may still have a high risk of developing cardiovascular diseases. This is where inhibiting the action of the PCSK9 gene can play an important role in preventing these diseases. Through specific medications that block the abnormal function of PCSK9, it is possible to significantly reduce LDL cholesterol levels in the blood and decrease the risk of serious cardiovascular events such as heart attack, stroke, and other problems related to the heart and blood vessels.The task of finding PCSK9 in our vast genome
In 2021, the Laboratory of Genetics and Molecular Cardiology conducted research that assessed factors already known to be associated with cardiovascular diseases. As in other studies, researchers found a relationship between LDL cholesterol and total cholesterol. Other factors, such as sex, age, race, and smoking – a significant lifestyle-related risk factor – were also evaluated, but showed no relationship with abnormal PCSK9 levels in the blood. The most significant discovery of this study was found in the genome. Researchers focused on evaluating the genome locations where PCSK9 abnormalities occur, and for that, they used a technique called co-localization. This technique is commonly used in genetic studies to determine if different genetic variants are associated with the same genome region. Co-localization analysis can lead to the identification of genes or variants that cause a particular condition or characteristic. Scientists observed that if something was affecting changes in patients’ cholesterol levels, the answer could be found in chromosomes 1, 7, 12, and 14 – which are complex structures made of a single molecule of highly coiled DNA. They also identified four potentially related genes. These findings bring us closer to the possibility of more precise treatments and the identification of genetic factors has become pivotal in establishing new disease predictors and identifying new targets for drugs.Other sources
Abifadel, Marianne, Mathilde Varret, Jean-Pierre Rabès, Delphine Allard, Khadija Ouguerram, Martine Devillers, Corinne Cruaud, et al. 2003. “Mutations in PCSK9 Cause Autosomal Dominant Hypercholesterolemia.” Nature Genetics 34 (2): 154–56. https://doi.org/10.1038/ng1161.
Aquino, Estela M. L., Sandhi Maria Barreto, Isabela M. Bensenor, Marilia S. Carvalho, Dóra Chor, Bruce B. Duncan, Paulo A. Lotufo, et al. 2012. “Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): Objectives and Design.” American Journal of Epidemiology 175 (4): 315–24. https://doi.org/10.1093/aje/kwr294.
Langlois, Michel R., and Victor H. Blaton. 2006. “Historical Milestones in Measurement of HDL-Cholesterol: Impact on Clinical and Laboratory Practice.” Clinica Chimica Acta, Special issue celebrating the 50th anniversary of Clinica Chimica Acta, 369 (2): 168–78. https://doi.org/10.1016/j.cca.2006.01.031.
Momtazi-Borojeni, Amir Abbas, Sarvenaz Sabouri-Rad, Antonio M Gotto Jr, Matteo Pirro, Maciej Banach, Zuhier Awan, George E Barreto, and Amirhossein Sahebkar. 2019. “PCSK9 and Inflammation: A Review of Experimental and Clinical Evidence.” European Heart Journal – Cardiovascular Pharmacotherapy 5 (4): 237–45. https://doi.org/10.1093/ehjcvp/pvz022.
Siri-Tarino, Patty W., and Ronald M. Krauss. 2016. “The Early Years of Lipoprotein Research: From Discovery to Clinical Application.” Journal of Lipid Research 57 (10): 1771–77. https://doi.org/10.1194/jlr.R069575.
Soutar, Anne K. 2011. “Unexpected Roles for PCSK9 in Lipid Metabolism.” Current Opinion in Lipidology 22 (3): 192. https://doi.org/10.1097/MOL.0b013e32834622b5.
Spolitu, Stefano, Wen Dai, John A. Zadroga, and Lale Ozcan. 2019. “Proprotein Convertase Subtilisin/Kexin Type 9 and Lipid Metabolism.” Current Opinion in Lipidology 30 (3): 186. https://doi.org/10.1097/MOL.0000000000000601.